AB-16B5 – inhibition of EMT
Alethia is developing an inhibitor of EMT for the treatment of invasive carcinomas. AB-16B5 is a humanized IgG2 antibody against an EMT-inducing form of secreted clusterin (sCLU). AB-16B5 very efficiently blocks EMT and pre-clinical data indicate that animals xenografted with various carcinoma cells show an increase of response to standard of care chemotherapy as well as a reduction of invasion.
Background and rationale
The molecular mechanisms responsible for the occurrence of metastatic cancer are beginning to be elucidated with the identification of key regulators. Increasing evidence points to tumor cell epithelial-to-mesenchymal transition (EMT) as an important contributing process to metastatic evolution. The occurrence of EMT during tumor progression permits epithelial tumor cells, that are noninvasive and non-metastatic, to move from the primary tumor, invade the surrounding tissue, enter the bloodstream, and finally disseminate to, and proliferate at, secondary sites. In addition, epithelial cancer cells that undergo EMT adopt a behavior that is very similar to cancer stem cells (CSCs) including an inherent resistance to chemo- and radiotherapy. Thus, the discovery of proteins that directly participate and promote EMT may represent new targets to increase the sensitivity of tumors to chemotherapy and prevent the spread of primary tumors cells to other sites.
Clusterin is a critical factor for stimulating EMT
A secreted protein termed clusterin (sCLU), was found to be stimulated during EMT and can, on its own, promote the EMT process. For example, epithelial cancer cells exposed to sCLU adopt a mesenchymal cell morphology, display increased mobility, and loose the expression of epithelial cell adhesion molecules such as E-cadherin. Importantly, over-expression of sCLU occurs in several malignancies including, prostate, bladder, kidney, breast, colon, and lung cancer. Furthermore, the level of expression of tumor-associated sCLU increases with disease progression and this expression in tumors contributes to the proliferation and survival of cancer cells. Thus, this role in malignancy combined with our novel finding of its role as a potent EMT inducer, imply that an inhibitor of sCLU represents a potential strategy for reducing or preventing tumor invasion as well as increasing the sensitivity of chemotherapy.
AB-16B5: a novel inhibitor of EMT
A domain in sCLU was identified that confers the EMT-inducing activity and we identified antibodies that specifically interact with this domain to inhibit this activity. The lead antibody in this program is designated AB-16B5, a humanized IgG2 that blocks EMT in cancer cells in vitro and prevents tumor invasion in vivo in animals models of metastasis. Human xenograft animal studies using prostate and pancreatic cancer tumors showed that blocking the activity of tumor-associated sCLU resulted in the increased response to standard chemotherapeutic drugs such as docetaxel and gemcitabine as measured by a significant reduction in tumor growth. Strikingly, AB-16B5 treatment alone reduced the growth of the tumors and animals treated with AB-16B5 showed an increase in survival. These findings and the results from several additional in vivo studies collectively showed that inhibition of tumor-associated sCLU with AB-16B5 increased the response to chemotherapy and reduced tumor invasion and tumor growth.
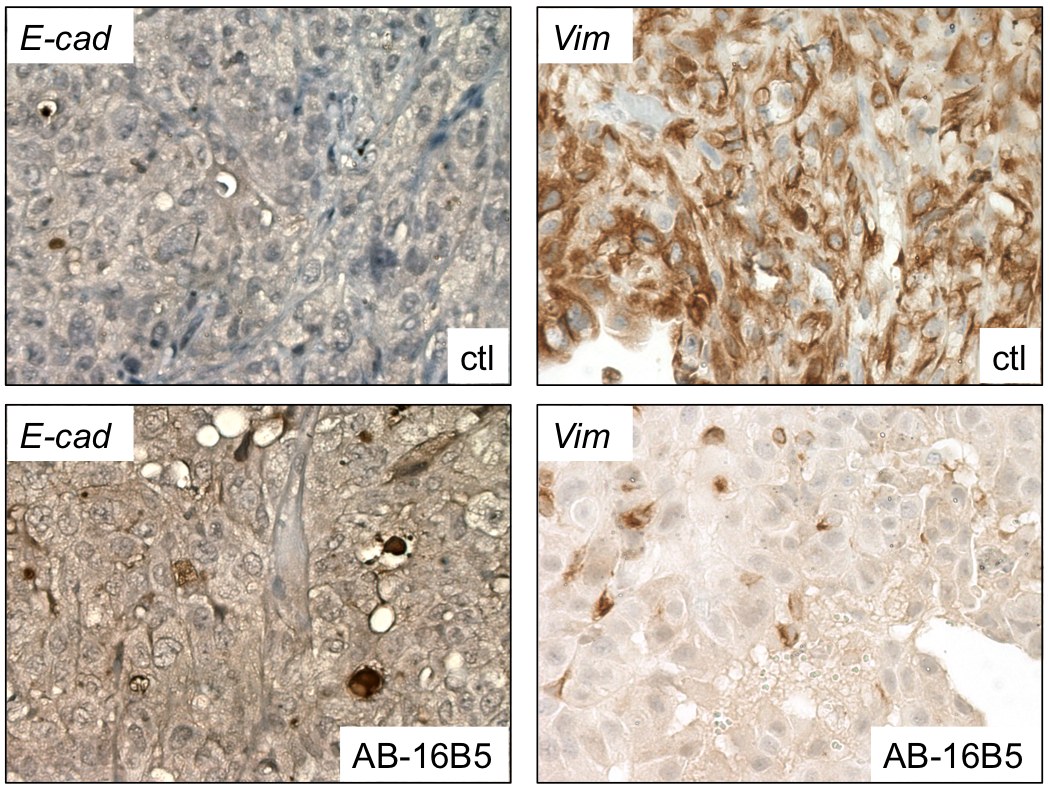
In agreement with these efficacy results, analysis by immunohistochemistry of the prostate tumor xenografts harvested from mice treated with AB-16B5 showed that the expression of E-cadherin, a marker of epithelial cells, was increased and vimentin, a marker of mesenchymal cells, was decreased (see below). Similar results were observed in pancreatic and lung tumors treated with AB-16B5.
Examination of CD44 expression in these tumors revealed that AB-16B5 caused a severe decrease in the level of this protein. CD44 was shown to be associated with the presence of CSCs and this finding supports the idea that there is a direct link between EMT and the formation of CSCs in tumors. Indeed, although it is not known if blocking sCLU with AB-16B5 has a direct effect on the renewal of CSCs, this results suggests that AB-16B5 might have activity to reduce the number of CSCs in tumors.
AB-16B5 was also evaluated in lung cancer and was found to have similar in vivo efficacy in combination with docetaxel. In parallel, we established that sCLU directly influenced the expression and activation of epidermal growth factor receptor (EGFR). Briefly, we found that incubation of NSCLC cell lines with AB-16B5 maintained high E-cadherin expression in addition to causing an increase in EGFR levels and phosphorylation status. These observations imply that these cancer cells would show increased response to EGFR inhibitors including tyrosine kinase inhibitors and monoclonal antibodies against EGFR.
Taken together, these exciting results completely validate the therapeutic strategy of targeting sCLU to block EMT. In fact, AB-16B5 represents one of the rare EMT inhibitors that demonstrated inhibition of EMT in vivo in human models of cancer.